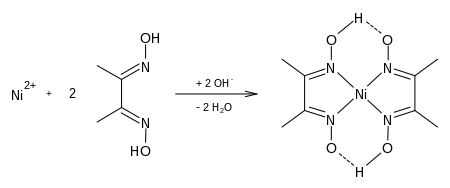
Synthesis Of Ni Dmg Complex
| Physical Chemistry Virtual Lab Physical chemistry (also called physicochemistry) is the explanation of macroscopic, microscopic, atomic, subatomic, and particulate phenomena in chemical systems in terms of physical concepts; sometimes using the principles, practices and concepts of physics like thermodynamics, quantum chemistry, statistical mechanics and dynamics. Spectrophotometry Cryoscopy Ebullioscopy EMF measurement Determination of Viscosity of Organic Solvents Adsorption Isotherm Verification of Tafel Equation Determination of Viscosity Average Molecular Weight of Polymer Calorimetry -Water equivalent Calorimetry Calorimetry -Heat of Neutralization |
| Organic Chemistry Virtual Lab Organic chemistry is a discipline within chemistry which involves the scientific study of the structure, properties, composition, reactions, and preparation (by synthesis or by other means) of chemical compounds that contain carbon. Detection of Functional Groups Detection of Elements: Lassaigne鈥檚 Test Separation of Compounds Using Column Chromatography Purification by Fractional distillation/crystallisation Purification by Steam distillation/crystallisation Laser Flash Photometer Organic Preparations - Allylation of Isatin Estimation of Aspirin Estimation Of Glucose Calculation of 位max of Organic Compounds Using Woodward Fieser Rules |
| Inorganic Chemistry Virtual Lab Inorganic chemistry is the branch of chemistry concerned with the properties and behavior of inorganic compounds. This field covers all chemical compounds except the myriad organic compounds (carbon based compounds, usually containing C-H bonds). Water analysis-Determination of Physical parameters Water analysis-Determination of Chemical parameters Acid Base Titration Gravimetric Estimation of Barium Gravimetric Estimation of Nickel Crystal Field Theory Group Theory Alloy Analysis (Brass) Soil Analysis-Determination of Specific conductivity of Soil Soil Analysis-Determination of pH of Soil |
| Advanced Analytical Chemistry Virtual Lab Analytical chemistry is the branch of chemistry concerned with studying the properties of materials and development of tools used to analyze materials. It is the science of sampling, defining, isolating , concentrating and preserving samples. Soil Analysis-Determination of Available Organic Carbon content in the Soil Soil Analysis-Determination of Available Nitrogen content in the Soil by Kjeldahl method Soil Analysis-Determination of Available Phosphorus content in the Soil by Bray's method Electrogravimetric Estimation of Metals Estimation of Phosphate Content in Soft Drinks Flame Photometry Polarography - Determination of Unknown Concentration of Cadmium Polarography - Determination of Unknown Concentration of Vitamin C |
Continuing on with the analysis of the Ni-en complex as found in question #3, 40 mL of dimethylglyoxime, DMG, solution was added to the titrated nickel-en complex solution. The resulting beautiful red complex, Ni(DMG) 2 was collected by filtration and found to weigh 0.469 g. What is the mass (in grams) of Ni in the Ni-en complex analyzed? Dichlorobis(ethylenediamine)nickel(II) is the inorganic compound with the formula Ni 2 Cl 4 (en) 4, where en = ethylenediamine.It is a salt of the coordination complex Ni 2 Cl 2 (en) 4 2+ with chloride counterions. This blue solid is soluble in water and polar organic solvents. Further, the Ni(dedtchl complex did not interact with aqueous en to give rise to Ni(enh12+ complex. But the mixed ligand complex Ni(dedtc)(DMG)l reacted with en to give rise to Ni(enhf+ ion. It is also interesting to note that of the four dithiocarbamate complexes, only two, viz.
| Physical Chemistry Virtual Lab Physical chemistry (also called physicochemistry) is the explanation of macroscopic, microscopic, atomic, subatomic, and particulate phenomena in chemical systems in terms of physical concepts; sometimes using the principles, practices and concepts of physics like thermodynamics, quantum chemistry, statistical mechanics and dynamics. Spectrophotometry Cryoscopy Ebullioscopy EMF measurement Determination of Viscosity of Organic Solvents Adsorption Isotherm Verification of Tafel Equation Determination of Viscosity Average Molecular Weight of Polymer Calorimetry -Water equivalent Calorimetry Calorimetry -Heat of Neutralization |
| Organic Chemistry Virtual Lab Organic chemistry is a discipline within chemistry which involves the scientific study of the structure, properties, composition, reactions, and preparation (by synthesis or by other means) of chemical compounds that contain carbon. Detection of Functional Groups Detection of Elements: Lassaigne鈥檚 Test Separation of Compounds Using Column Chromatography Purification by Fractional distillation/crystallisation Purification by Steam distillation/crystallisation Laser Flash Photometer Organic Preparations - Allylation of Isatin Estimation of Aspirin Estimation Of Glucose Calculation of 位max of Organic Compounds Using Woodward Fieser Rules |
| Inorganic Chemistry Virtual Lab Inorganic chemistry is the branch of chemistry concerned with the properties and behavior of inorganic compounds. This field covers all chemical compounds except the myriad organic compounds (carbon based compounds, usually containing C-H bonds). Water analysis-Determination of Physical parameters Water analysis-Determination of Chemical parameters Acid Base Titration Gravimetric Estimation of Barium Gravimetric Estimation of Nickel Crystal Field Theory Group Theory Alloy Analysis (Brass) Soil Analysis-Determination of Specific conductivity of Soil Soil Analysis-Determination of pH of Soil |
| Advanced Analytical Chemistry Virtual Lab Analytical chemistry is the branch of chemistry concerned with studying the properties of materials and development of tools used to analyze materials. It is the science of sampling, defining, isolating , concentrating and preserving samples. Soil Analysis-Determination of Available Organic Carbon content in the Soil Soil Analysis-Determination of Available Nitrogen content in the Soil by Kjeldahl method Soil Analysis-Determination of Available Phosphorus content in the Soil by Bray's method Electrogravimetric Estimation of Metals Estimation of Phosphate Content in Soft Drinks Flame Photometry Polarography - Determination of Unknown Concentration of Cadmium Polarography - Determination of Unknown Concentration of Vitamin C |
- Pelagia Research Library Der Chemica Sinica, 2016, 7(3):83-86 ISSN: 0976-8505 CODEN (USA) CSHIA5 83 Pelagia Research Library Synthesis and characterization of mixed ligand complexes of Cr (II), Cu (II) and Ni (II) metals containing DMG and dicarboxylic acids as ligands.Sonaji V. Gayakwad, 1Satish B. Maulage and 2Mahendra N.
- Aug 24, 2011 A new family of low-coordinate nickel imides supported by 1,2-bis(di-tert-butylphosphino)ethane was synthesized.The oxidation of nickel(II) complexes led to the formation of both aryl- and alkyl-substituted nickel(III) imides, and examples of.

Ni Dmg Complex
Jul 18, 2019 The only reason for precipitation of Nickel in DMG is due to formation of an insoluble complex Ni (dmg)2 In basic medium the hydroxy group ( -OH) of dimethylglyoxime is neutralised and as a result it gets converted to O- which is favorable for H. Jan 09, 2008 Ni(DMG)2(s) is a red precipitate. As you can see nickel has not been oxidized, nickel is not oxidized as the Ni2+ ion only forms a complex. DMG has to be in an alcohol solution because It is the conjugate base, not dmgH2 itself, that forms the complexes. Knights of the old republic 2 free download mac fonts.